
Medical Effects: "Hut-Lung" Case Study
Environ Health Perspect. 2006 May; 114(5): 759–762.
Published online 2006 January 24. doi: 10.1289/ehp.8489.
Copyright This is an Open Access article: verbatim copying and redistribution of
this article are permitted in all media for any purpose, provided this
notice is preserved along with the article's original DOI.
Environmental Medicine
Case Report: A Case of Wood-Smoke–Related Pulmonary Disease
Janet V. Diaz,1 Jonathan Koff,2 Michael B. Gotway,3 Stephen Nishimura,3 and John R. Balmes1
1 Department of Medicine, Division of Pulmonary and Critical Care Medicine,
2 Department of Radiology and
3 Department of Pathology, San Francisco General Hospital, University of
California at San Francisco, San Francisco, California, US
Received July 12, 2005; Accepted January 23, 2006.
|
Abstract:
Context. Biomass
serves as a major fuel source for more than 50% of the world’s population.
The global burden of disease attributed to indoor air pollution from
biomass combustion accounts for approximately 3% of worldwide
disability-adjusted life-years lost. This is due to pneumonia in
children and chronic obstructive pulmonary disease and lung cancer in
women.
Case Presentation. A
53-year-old man from Mexico was referred to the pulmonary clinic for
evaluation of chronic productive cough and pulmonary nodules. In his
youth, he worked at a charcoal plant in Mexico, where he burned wood
and was exposed to massive amounts of smoke. His evaluation revealed
thickened bronchovascular bundles with nodules on thoracic computed
tomography, dark black plaques in large airways on bronchoscopy, and
carbon-laden macrophages and fibrotic scars on lung biopsy.
Discussion. The
patient was diagnosed with “hut lung,” a term that refers to the
noninfectious, nonmalignant respiratory manifestations of chronic,
high-level exposures to biomass smoke. This is the first reported case
of hut lung associated with charcoal production. This case highlights
that histopathologic abnormalities of the lung parenchyma may be
present in patients with only mild symptoms and that clinical
progression is likely a function of both the duration and intensity of
exposure.
Relevance to Clinical Practice. As
residents of lesser developed countries continue to be exposed to high
levels of biomass smoke at work or at home and continue to immigrate to
developed countries, it is important that health care providers in
developed countries be aware of biomass-smoke–related pulmonary disease.
Keywords: biomass combustion, domestically acquired particulate lung disease, hut lung, indoor air pollution, wood smoke
Case Presentation
A
53-year-old man from Mexico was referred to the San Francisco General
Hospital chest clinic for the evaluation of a chronic cough and
pulmonary nodules. His respiratory symptoms began in 1985 as an
intermittent cough. The cough gradually progressed over the years and
was now chronic, productive of yellow sputum, and associated with mild
dyspnea. In 1997, he was diagnosed with asthma in Mexico and treated
with inhaled bronchodilators with minimal relief. In 2004, he
immigrated to the United States and was seen by a primary care provider
for these symptoms and treated with a steroid inhaler without relief.
At that evaluation, a chest radiograph was performed (Figure 1A), and he was then referred to a chest clinic.
At
his chest clinic visit, he denied any current fevers, chills, sweats,
weight loss, eye pain, arthralgias, rash, or sinusitis. He had no pets
or mold at home, no recent travel, and no environmental tobacco smoke
exposures. He reported no other medical conditions. He denied any
tobacco, alcohol, or illicit drug use.
His occupational history
was significant for work at a charcoal production plant in Mexico when
he was 18–26 years of age. He spent 10–12 hr/day burning wood to make
charcoal, and he stood very near the fire. He described the environment
as being very smoky. He did not use any protective respiratory masks.
He denied mining, construction, or asbestos-related occupations. For
the past year, he had been working in a restaurant as a dishwasher.
On
physical examination, he appeared healthy without any signs of distress
and had normal vital signs and an oxygen saturation of 97%. The
remainder of the examination was normal except for bilateral forced
expiratory wheezes. He had no signs of pulmonary hypertension, right
heart failure, or clubbing. Routine laboratory tests revealed a
complete blood count and blood chemistry panel that were normal. Three
sputum samples for mycobacteria had no growth at 56 days. Serologies
for fungal diseases were negative.
We performed thoracic
high-resolution computed axial tomography (HRCT), using 7 mm helical
technique after intravenous contrast administration followed by
high-resolution imaging, using 1 mm collimation every 1 cm from lung
apex to base (Figure 1B–D).
Pulmonary function test results were as follows: forced vital capacity
(FVC), 3.46 L (91%); forced expiratory volume in 1 sec (FEV1), 2.31 L (74%); FEV1:FVC
ratio, 67; total lung capacity, 5.27 L (95%); residual volume, 1.57 L
(91%); diffusion capacity for carbon monoxide, 23.1 mL/mmHg/min (81%);
and diffusion capacity for CO corrected for total lung capacity by
single breath, 4.61 mL/mmHg/L (87%). Arterial blood gases were normal.
The
patient underwent bronchoscopy for the suspected diagnosis of
sarcoidosis. Bronchoscopy demonstrated a large black and gray plaque in
the left mainstem bronchus (Figure 2)
and multiple smaller gray and black deposits in the left upper lobe,
left lower lobe, and right lower lobe. Transbronchial biopsies were
obtained (Figure 3). Bronchoalveolar lavage fluid showed normal cell differential counts.
The patient was diagnosed with “hut lung,” or domestically acquired particulate lung disease (Gold et al. 2000; Grobbelaar and Bateman 1991).
Discussion
The
term “hut lung” has been used to describe a wide spectrum of clinical
manifestations including chronic bronchitis (CB), chronic obstructive
pulmonary disease (COPD), and interstitial lung disease associated with
high level exposures to biomass smoke. This is the first reported case
of hut lung associated with charcoal production. It highlights the
characteristic findings of hut lung that have been reported in previous
case series of women from the developing world who cook with biomass
indoors (Gold et al. 2000; Grobbelaar and Bateman 1991; Ozbay et al. 2001; Sandoval et al. 1993).
This case clearly demonstrates that physiologic, radiographic, and
histopathologic abnormalities may persist years after removal from
exposure. Hut lung is likely underdiagnosed because those at risk have
poor access to health care. This raises the importance of recognizing
risk for this disease among biomass-smoke–exposed populations.
Biomass
is any material derived from living or recently living material,
including animal dung, twigs, grass, crop wastes, wood, and charcoal.
More than half of the world’s population uses biomass as a major source
of energy for cooking, baking, and heating. This occurs predominantly
in rural areas of lesser developed countries where biomass is burned
indoors. Because homes are poorly ventilated and this fuel source is
inefficient, requiring fires to be kept going for many hours a day,
women and their infant children are exposed to years of daily smoke (Bruce et al. 2000; Ezzati and Kammen 2002; Manuel 2003).
Biomass
combustion releases smoke that contains particulate matter (PM), CO,
nitrogen oxides, formaldehyde, and polyaromatic hydrocarbons (Boman et al. 2003; Zelikoff et al. 2002). Indoor biomass combustion creates massive amounts of indoor air pollution. Measurements of 24-hr mean indoor levels of PM10 (particles with mass median aerodynamic diameter of < 10 μm) have been reported between 300 and 30,000 μg/m3 and CO between 2 and 500 ppm: these levels are 2–200 times higher than
the U.S. Environmental Protection Agency regulations for outdoor air
pollutants (Bruce et al. 2000; Ezzati and Kammen 2002; Manuel 2003). PM10 can bypass the filtering system of the nasal and oral cavity to either
deposit on the mucosa of large- and medium-sized airways (coarse PM) or
deposit deep in the alveoli (fine PM), and thus is able to affect
respiratory health (Balmes and Tager 2000). Exposure to high levels of
outdoor PM10 is independently related to lung cancer and cardiopulmonary mortality [World Health Organization (WHO) 2002].
There
are limited data on the mechanisms by which biomass smoke causes
chronic pulmonary disease. Both macrophage dysfunction and increased
activity of matrix metalloproteinase (MMP) have been reported. Rabbits
exposed to acute wood smoke had impaired macrophage phagocytic
function, surface adherence (Fick et al. 1984), and reduced bacterial clearance (Zelikoff et al. 2002).
Rats exposed to chronic wood smoke developed mild bronchiolitis with
epithelial cell hyperplasia and hypertrophy, alveolar septal
thickening, and mild emphysema (Lal et al. 1993).
Bronchoalveolar lavage samples from human subjects with COPD associated
with wood-smoke exposure demonstrated significantly higher MMP
activity, specifically pro-MMP-2, pro-MMP-9, and MMP-9, and gene
expression of MMP-2 and MMP-12, when compared with healthy controls (Montano et al. 2004).
Controlled animal and human exposures to concentrated ambient
particulates have demonstrated induction of pulmonary inflammation (Saldiva et al. 2002).
There
is strong epidemiologic evidence that biomass smoke is associated with
the development of CB and COPD. The prevalence rates of CB in
communities exposed to indoor biomass smoke have been reported to be
high (Albalak et al. 1999; Behera and Jindal 1991; Golshan et al. 2002; Pandey 1984; Pandey et al. 1985; Perez-Padilla et al. 1996).
In rural Nepal, the prevalence rate of CB was 19.8% in nonsmoking women
who spent more than 4 hr/day near the fireplace, and in rural Bolivia,
the prevalence rate was 23% in a nonsmoking community that cooked
primarily indoors with cow dung (Albalak et al. 1999; Pandey et al. 1985).
Case–control studies have demonstrated that wood smoke exposure is an
independent risk factor for the development of COPD, with odds ratios
in the range of 4–15 (Dennis et al. 1996; Perez-Padilla et al. 1996). In a cohort of Colombian women, the population attributable risk was 50% (Dennis et al. 1996).
In a 2002 WHO report (WHO 2002),
the global burden of disease attributed to indoor air pollution from
biomass combustion accounted for 2.7% of worldwide disability-adjusted
life-years lost (Ezzati and Kammen 2002; WHO 2002), placing indoor smoke as the second largest environmental contributor to poor health, behind unsafe water and sanitation (WHO 2002).
Indoor smoke accounts for 4–5% of global mortality, with 56% of these
deaths due to childhood acute lower respiratory infections and the
remainder due to COPD and lung cancer, primarily in women (Ezzati and Kammen 2002; WHO 2002). As the global burden of COPD continues to rise, projected to rank as the fifth most burdensome condition by 2020 (Pauwels et al. 2001), and poverty persists, we can assume the burden of disease due to biomass combustion will also continue to rise (Bruce et al. 2000).
Patients
with hut lung can present with a wide spectrum of symptoms, ranging
from quite benign to severe. This case demonstrates that a productive
cough and mild dyspnea can persist for years after removal of the
exposure. In the first published series of 25 rural South African
women, most of the women were asymptomatic, and the remainder either
had acute cough or a chronic productive cough (Grobbelaar and Bateman 1991).
In contrast, in a later series of 30 rural Mexican women with pulmonary
hypertension and cor pulmonale, all of the women had dyspnea and nearly
all had a productive cough. Other common findings included cyanosis
(63%), crackles (70%), hepatomegaly (60%), and edema (73%) (Sandoval et al. 1993).
In the above series, because the mean age of the Mexican women was
higher than the South African women (63 years vs. 43 years,
respectively), it is likely that they had substantially greater
cumulative exposures to smoke. This suggests that early disease can be
masked by the lack of or nonspecific nature of symptoms and that
duration of exposure is correlated with the severity of disease.
Pulmonary
function tests also demonstrate a wide spectrum of abnormalities. The
present case demonstrates that airflow obstruction can be one of the
initial physiologic changes. In the previous case series, most South
African women had mild and moderate airway obstruction (16 of 22) and a
decreased diffusion capacity (13 of 17), whereas the remainder had
normal (5 of 22) or mild restriction (1 of 22) (Grobbelaar and Bateman 1991).
Although most of the Mexican women also had obstruction (23 of 30),
many also had mild restriction or a mixed picture (18 of 30). Arterial
blood gases demonstrated severe hypoxemia in all patients and some with
hypercapnea (12 of 30) (Sandoval et al. 1993).
Diffusing capacity was not measured. This suggests that early disease
can be masked by normal pulmonary function tests, that airflow
obstruction and impaired diffusion capacity are the initial physiologic
changes, and that at late stages mild restriction and gas exchange
abnormalities develop. Deterioration of pulmonary function also seems
to be correlated with the duration and intensity of exposure.
Characteristic
findings have been reported on bronchoscopy and bronchoalveolar lavage.
In this case, gross inspection of the large airways showed dark blue
stains that were similar to the airway findings described in the
Mexican series (Sandoval et al. 1993).
Our case also revealed normal lavage fluid cell differential counts and
carbon-laden alveolar macrophages, as was reported in the South African
series (Grobbelaar and Bateman 1991).
The
characteristic but nonspecific plain chest radiographic findings of hut
lung are diffuse pulmonary nodules. Our case illustrates that on HRCT
these nodules are distributed along the bronchovascular bundles and can
coexist with mediastinal lymphadenopathy. In a study from Turkey (Kara et al. (2003),
a comparison of HRCT scans of 60 nonsmoking women with at least 10
years of biomass exposure with nonexposed controls showed significantly
more of the following abnormalities: reticulation, peribronchovascular
thickening, and nodular and ground glass opacities. The asymptomatic
subjects with exposure had significantly more ground-glass opacities
and less bronchiectasis than those with symptoms (Kara et al. 2003).
These data suggest that radiographic abnormalities are seen early in
the disease, even in asymptomatic or mildly symptomatic individuals,
and persist years after removal from exposure.
Lung
histopathology obtained by either transbronchial or open lung biopsy is
the gold standard for the diagnosis of hut lung. This case illustrates
the classic findings of carbon pigment deposition around terminal
bronchioles, dust macules, and mixed dust fibrosis. In the South
African series, Grobbelaar and Bateman (1991) described three patterns: isolated carbon deposition (12 of 25),
macules as carbon pigment within focal collections of dust laden
macrophages (6 of 25), and mixed dust fibrosis as stellate interstitial
fibrous lesions (7 of 25). In the Mexican series, Sandoval et al. (1993) observed mixed dust fibrosis on lung biopsies, whereas they observed CB
on airway biopsies. The available data again suggest that
histopathologic changes are seen early in disease, even in asymptomatic
or mildly symptomatic individuals, and persist years after removal from
exposure.
Conclusion: Hut
lung appears to represent the non-infectious, nonmalignant respiratory
manifestations of chronic, high level exposures to biomass smoke. There
is strong evidence that chronic exposure to high levels of smoke from
the combustion of biomass indoors is a risk factor for the development
of CB and COPD in women of the developing world and growing evidence
that an interstitial lung disease characterized by carbon deposition,
dust macules, and mixed dust fibrosis also exists. This case is the
first report of hut lung associated with charcoal production. The
literature suggests that hut lung is likely to be part of a spectrum of
disease in which intensity and duration of exposure affects the disease
manifestation. Patients with early disease are either asymptomatic or
present with cough or mild COPD, but radiographically and
pathologically may have significant abnormalities, including fibrosis.
Research is needed to better characterize disease mechanism,
progression, and interventions for prevention and treatment. As
residents of lesser developed countries continue to be exposed to high
levels of biomass smoke at home or at work and continue to immigrate to
developed countries, it is important that health care providers in
developed countries learn to recognize this clinical entity.
- References
- Albalak R, Frisancho AR, Keeler GJ. Domestic biomass fuel combustion and chronic bronchitis in two rural Bolivian villages. Thorax. 1999;54(11):1004–1008. [PubMed]
- Balmes
J, Eisner M, Tager I. 2005. Air pollution. In: Textbook of Respiratory
Medicine (Murray JF, Nadel JA, eds). Philadelphia:W.B. Saunders,
1800–1813.
- Behera D, Jindal SK. Respiratory symptoms in Indian women using domestic cooking fuels. Chest. 1991;100(2):385–388. [PubMed]
- Boman
BC, Forsberg AB, Jarvholm BG. Adverse health effects from ambient air
pollution in relation to residential wood combustion in modern society. Scand J Work Environ Health. 2003;29(4):251–260. [PubMed]
- Bruce
N, Perez-Padilla R, Albalak R. Indoor air pollution in developing
countries: a major environmental and public health challenge. Bull WHO. 2000;78(9):1078–1092. [PubMed]
- Dennis
RJ, Maldonado D, Norman S, Baena E, Martinez G. Wood smoke exposure and
risk for obstructive airways disease among women. Chest. 1996;109(1):115–119. [PubMed]
- Ezzati
M, Kammen DM. The health impacts of exposure to indoor air pollution
from solid fuels in developing countries: knowledge, gaps, and data
needs. Environ Health Perspect. 2002;110:1057–1068. [PubMed]
- Fick
RB Jr, Paul ES, Merrill WW, Reynolds HY, Loke JS. Alterations in the
antibacterial properties of rabbit pulmonary macrophages exposed to
wood smoke. Am Rev Respir Dis. 1984;129(1):76–81. [PubMed]
- Gold
JA, Jagirdar J, Hay JG, Addrizzo-Harris DJ, Naidich DP, Rom WN. Hut
lung. A domestically acquired particulate lung disease. Medicine (Baltimore). 2000;79(5):310–317. [PubMed]
- Golshan M, Faghihi M, Marandi MM. Indoor women jobs and pulmonary risks in rural areas of Isfahan, Iran, 2000. Respir Med. 2002;96(6):382–388. [PubMed]
- Grobbelaar JP, Bateman ED. Hut lung: a domestically acquired pneumoconiosis of mixed aetiology in rural women. Thorax. 1991;46(5):334–340. [PubMed]
- Kara
M, Bulut S, Tas F, Akkurt I, Seyfikli Z. Evaluation of pulmonary
changes due to biomass fuels using high-resolution computed tomography. Eur Radiol. 2003;13(10):2372–2377. [PubMed]
- Lal
K, Dutta KK, Vachhrajani KD, Gupta GS, Srivastava AK.
Histomorphological changes in lung of rats following exposure to wood
smoke. Indian J Exp Biol. 1993;31(9):761–764. [PubMed]
- Manuel J. The quest for fire: hazards of a daily struggle. Environ Health Perspect. 2003;111:A28–A33. [PubMed]
- Montano
M, Beccerril C, Ruiz V, Ramos C, Sansores RH, Gonzalez-Avila G. Matrix
metalloproteinases activity in COPD associated with wood smoke. Chest. 2004;125(2):466–472. [PubMed]
- Ozbay B, Uzun K, Arslan H, Zehir I. Functional and radiological impairment in women highly exposed to indoor biomass fuels. Respirology. 2001;6(3):255–258. [PubMed]
- Pandey MR. Domestic smoke pollution and chronic bronchitis in a rural community of the Hill Region of Nepal. Thorax. 1984;39(5):337–339. [PubMed]
- Pandey MR, Regmi HN, Neupane RP, Gautam A, Bhandari DP. Domestic smoke pollution and respiratory function in rural Nepal. Tokai J Exp Clin Med. 1985;10(4):471–481. [PubMed]
- Pauwels
RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for
the diagnosis, management, and prevention of chronic obstructive
pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive
Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. [PubMed]
- Perez-Padilla
R, Regalado J, Vedal S, Pare P, Chapela R, Sansores R, et al. Exposure
to biomass smoke and chronic airway disease in Mexican women. A
case-control study. Am J Respir Crit Care Med. 1996;154(3 Pt 1):701–706. [PubMed]
- Saldiva
PH, Clarke RW, Coull BA, Stearns RC, Lawrence J, Murthy GG, et al. Lung
inflammation induced by concentrated ambient air particles is related
to particle composition. Am J Respir Crit Care Med. 2002;165(12):1610–1617. [PubMed]
- Sandoval
J, Salas J, Martinez-Guerra ML, Gomez A, Martinez C, Portales A, et al.
Pulmonary arterial hypertension and cor pulmonale associated with
chronic domestic wood smoke inhalation. Chest. 1993;103(1):12–20. [PubMed]
- WHO 2002. World Health Report 2002. Reducing Risks, Promoting Healthy Life. Geneva:World Health Organization.
- Zelikoff JT, Chen LC, Cohen MD, Schlesinger RB. The toxicology of inhaled wood smoke. J Toxicol Environ Health B Crit Rev. 2002;5(3):269–282. [PubMed]
Figure 1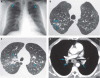
Chest radiograph (
A) and HRCT images (
B–D) of patient’s lungs. (
A)
Frontal chest radiograph showing mild symmetric linear and reticular
opacities (arrows) in the upper lobes bilaterally; these opacities are
associated with bilateral hilar
(more ...)Figure 2 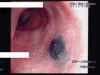
Bronchoscopy
demonstrated a black and gray plaque in the left mainstem bronchus.
White bars cover the patient’s name and medical record number.
Figure 3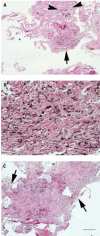
Histopathologic features of transbronchial biopsies. (
A)
Anthracotic pigment accumulating along alveolar septae (arrowheads) and
within a pigmented dust macule (single arrow); bar = 200 μm. (
B) A high-power photomicrograph containing a
(more ...)
© 2007-2017 Clean Air Revival Home page at
http://BurningIssues.org
© 2007 Clean Air Revival Home page at
http://BurningIssues.org
Go to top



